Why Do Electrons Occupy Orbitals in a Specific Order?
Awesome — since you’re here, that means you’re not just trying to memorize the order of orbitals from a textbook 🧪 — you’re actually trying to understand why the heck electrons fill them in that specific weird-looking sequence like 1s, 2s, 2p, 3s, 3p, 4s… and not just in straight numerical order.
So let’s skip the jargon and get into it like we’re two friends nerding out about atoms — casually and clearly.
—
Why Do Electrons Occupy Orbitals in a Specific Order?
Okay, imagine electrons are tiny party guests — and orbitals are the rooms they’re allowed to hang out in inside an atomic “hotel.”
But here’s the twist: electrons are super picky. They don’t just enter any room randomly. They go by a super-specific checklist:
- They love low energy spots — the lower the better.
- They hate being in the same place as other electrons if they don’t have to.
- And they follow the rules of quantum mechanics (even if they don’t understand them either 😅).
That’s why they fill orbitals in a very specific order — to keep the energy of the atom as low and stable as possible.
Let’s break it down in human terms.
—

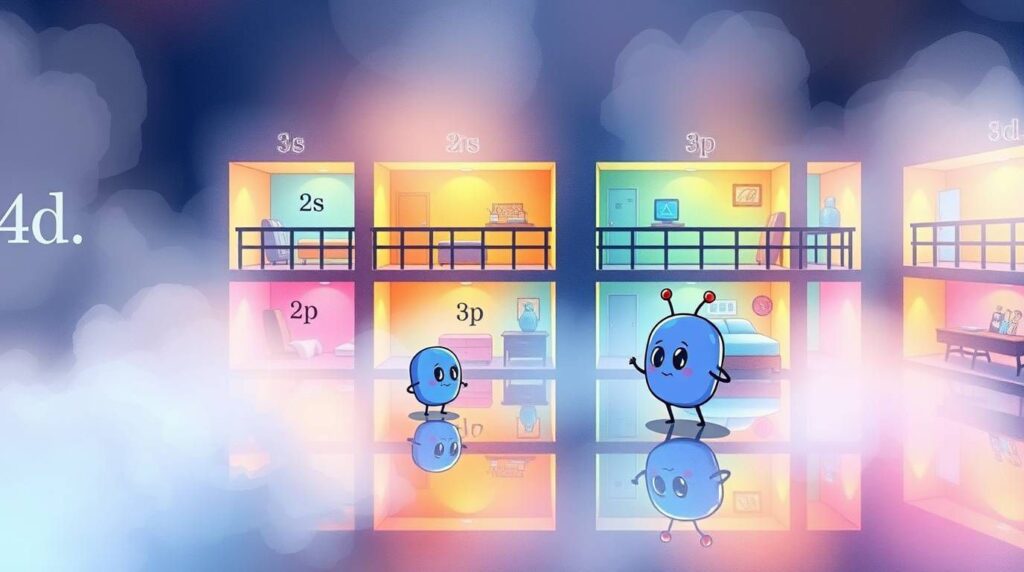


🎯 Step 1: What Are Orbitals? (continued)
Orbitals are like tiny regions around the nucleus of an atom where electrons are most likely to be found. They’re not actual orbits like planets — more like “zones” where an electron is vibing most of the time.
There are four main types of orbitals you’ll hear about:
- s-orbitals: shaped like spheres 🟢
- p-orbitals: kinda like dumbbells or figure-8s 🏋️
- d-orbitals: more complex — like clover shapes 🍀
- f-orbitals: super complex, just… chaos 💥
Each type has different energy levels and capacities. Electrons fill these orbitals in a very specific order — not by name (like 1s, 2s, 2p…) but by energy level.
—
📉 Step 2: Electrons Are Lazy (They Love Low Energy)
Electrons are like people looking for the coziest seat in a coffee shop. They’ll always go to the quietest, comfiest corner (aka, the lowest energy orbital) first. That’s why they fill the 1s orbital before 2s, and 2s before 2p.
This “filling order” is called the Aufbau principle (German for “building up”). It’s like saying: start from the basement and fill up to the rooftop — but only after every comfy room below is taken.
—
🌀 Step 3: Why Is the Order So Weird?
You’d expect electrons to go:
1s → 2s → 2p → 3s → 3p → 3d → 4s → 4p … right?
But nope. They go:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s…
Wait, why does 4s come before 3d? That sounds illegal 😩.
Here’s the secret: the energy of orbitals doesn’t increase perfectly by their number.
- The 4s orbital actually has slightly lower energy than the 3d orbital.
- So electrons sneak into the 4s orbital first — they’re all about minimal effort and chill energy.
Imagine going to the 4th floor of a building because the 3rd-floor room is weirdly more expensive. It’s exactly like that. Electrons are bargain hunters.
—
🧷 Step 4: The Rules They Follow
There are 3 big rules electrons follow when filling orbitals:
1. Aufbau Principle → Fill lowest energy first.
2. Pauli Exclusion Principle → Only 2 electrons per orbital max, and they must have opposite “spins.”
3. Hund’s Rule → Electrons like to spread out in orbitals of the same type before pairing up (because they’re antisocial little things).
This keeps the atom stable and helps prevent chaos. So yes, there’s structure to this madness!
—
🧠 TL;DR – Let’s Wrap It Up Like a Burrito:
- Electrons occupy orbitals in a specific order based on energy — not numerical sequence.
- They’re lazy and want the lowest energy possible (thanks, Aufbau Principle).
- That’s why weird stuff like 4s coming before 3d happens.
- They also follow social distancing rules (Hund’s Rule & Pauli’s Principle).
- It’s all about stability, low energy, and following physics’ funky rhythm.
Now if anyone asks why the electron configuration is so weird, you can just say:
“Because electrons are introverted energy snobs.”
And… you’d be right. 😄
—
📌 Disclaimer:
This easy version is meant to help you understand the concept better. If your exam or teacher expects a textbook explanation and you write this one instead, we’re not responsible if it affects your marks. Use this for understanding, not copy-pasting.
—
🔗 Related Articles from EdgyThoughts.com:
How Does Entropy Relate to Spontaneous Reactions?
https://edgythoughts.com/how-does-entropy-relate-to-spontaneous-reactions/
How Does Epigenetics Influence Gene Expression?
https://edgythoughts.com/how-does-epigenetics-influence-gene-expression/
🌐 External Resource:
Feeling brave? Here’s the official (but mind-bending) Wikipedia explanation:
https://en.wikipedia.org/wiki/Electron_configuration
—
What If We Could Alter Dreams in Real Time 2025
https://edgythoughts.com/what-if-we-could-alter-dreams-in-real-time-2025/
Can Probiotics Reduce Anxiety and Depression?
https://edgythoughts.com/can-probiotics-reduce-anxiety-and-depression/





