How does entropy relate to spontaneous reactions?
How Does Entropy Relate to Spontaneous Reactions?
As you’re here, that probably means you already peeked at the textbook explanation and went, “Yeah no thanks.” So instead of all that science-speak, let’s go full casual mode and really get what’s going on with entropy and those “spontaneous reactions.”
—
First: What Even Is Entropy?
Okay, imagine you clean your room. Everything’s in place. Tidy. Beautiful. Now give it a few days… and somehow your socks are in your bag, your notes are under your bed, and there’s a snack wrapper on your pillow. What happened?
That, my friend, is entropy.
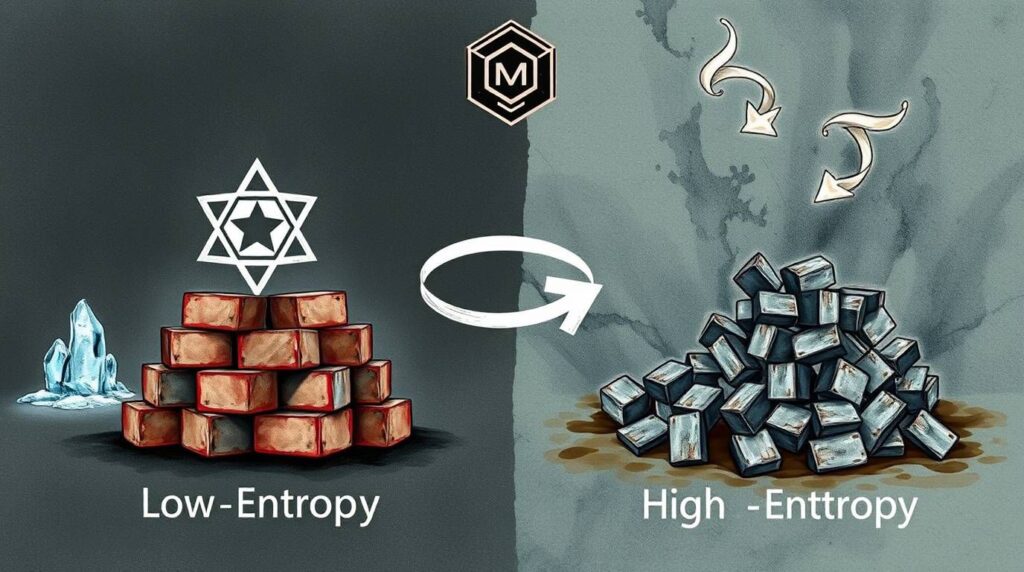
In science terms, entropy is just a fancy way of measuring disorder. The higher the entropy, the more chaotic or messy something is.
Nature has a habit — it loves to go from neat to messy. From organized to scattered. That’s why your room never magically cleans itself.
—



So What Are Spontaneous Reactions?
Spontaneous reactions are chemical reactions that just happen — no extra push needed. Like iron rusting. Or ice melting on a warm day. Or your banana turning brown when you forget it on the table for too long.
They don’t need your help. They’re just gonna do their thing when conditions are right.
But here’s the twist: “spontaneous” doesn’t always mean “fast.” Rusting takes time, but it’s still spontaneous. So don’t think of it like a sudden kaboom — think of it like a chill, inevitable process.
—
So How Does Entropy Tie Into This?
Alright — picture this:
When a chemical reaction happens, the particles (atoms and molecules) can get more spread out, more disordered, or more randomized. If that happens, the entropy of the system goes up.
And guess what? Reactions that increase entropy (aka create more disorder) are more likely to be spontaneous.
Let’s break it down with a few examples:
✅ Ice melting: Solid water (ice) has super organized molecules. But when it melts into liquid, those molecules move more freely = more disorder = entropy increases = spontaneous.
✅ Firecracker exploding: Before — one tiny, compact thing. After — boom, gas and heat everywhere = way more entropy = spontaneous reaction.
But hold on — it’s not just about entropy. There’s a partner in this called enthalpy (which is about energy). Together, they help decide if a reaction happens on its own.
And scientists put these two things together in something called the Gibbs Free Energy equation: ΔG = ΔH – TΔS
Don’t worry — you don’t have to memorize it. Just know this:
- If ΔG is negative → spontaneous reaction
- ΔS is entropy (disorder)
- ΔH is energy (heat released or absorbed)
So more entropy (higher ΔS) = more likely ΔG is negative = boom, spontaneous!
—
Why It Matters in 2025
Understanding entropy helps us design better batteries, predict how pollution spreads, develop efficient chemical processes, and even explain why your cold coffee gets warm sitting out (and never the other way around — sorry!).
In short, entropy tells us what’s gonna happen when we’re not looking.
—
TL;DR — Simple Version:
- Entropy = disorder or randomness.
- Nature likes things to be messy (aka high entropy).
- Spontaneous reactions are more likely when they increase entropy.
- Think melting ice, rusting metal, or mixing cream in coffee.
- It’s not just about being messy — energy and temperature also play a role.
—
📌 Disclaimer:
This easy version is meant to help you understand the concept better. If your exam or teacher expects a textbook explanation and you write this one instead, we’re not responsible if it affects your marks. Use this for understanding, not copy-pasting.
—
🔗 Related Articles from EdgyThoughts.com:
What If Atoms Could Remember Past Lives 2025
https://edgythoughts.com/what-if-atoms-could-remember-past-lives-2025
How Quantum Particles Decide Their Path 2025
https://edgythoughts.com/how-quantum-particles-decide-their-path-2025
🌐 External Resource:
Want the deep science behind it? Check out the Wikipedia page:
https://en.wikipedia.org/wiki/Entropy
—
What if night lasted for an entire year?
https://edgythoughts.com/what-if-night-lasted-for-an-entire-year/
Are Schools Teaching Emotional Intelligence Now?
https://edgythoughts.com/are-schools-teaching-emotional-intelligence-now/





